Open Access, Volume 9
An MDT treatment of extremely proptosis caused by the recurrent meningioma
Jinhua Liu1; Honglei Liu2*
1Department of Ophthalmology, Shaanxi Eye Hospital, Xi’an People’s Hospital (Xi’an Fourth Hospital), Affiliated People’s Hospital
of Northwest University, China.
2Shaanxi Eye Hospital, Xi’an People’s Hospital (Xi’an Fourth Hospital), Affiliated People’s Hospital of Northwest University, China.
Honglei Liu
Department of Ophthalmology, Shaanxi Eye Hospital, Xi’an People’s Hospital (Xi’an Fourth Hospital), Affiliated People’s Hospital of Northwest University, China.
Tel: +8613759926281; Email: davidhlliu@163.com
Received : January 30, 2023,
Accepted : February 22, 2023
Published : February 28, 2023,
Archived : www.jclinmedcasereports.com
Abstract
Meningiomas are the most common primary tumors of the central nervous system and are more common in female patients. Most meningiomas are benign, but the tumor infiltration can often lead to recurrence. We report a case of recurrent meningioma at the skull base in a juvenile, involving multiple sites including cranial and facial tissue, the prognosis is good after complete surgical resection.
Keywords: Meningiomas; Recurrent; Surgical resection; Reconstruction.
Abbreviations: MDT: Multi-Disciplinary Team; RAPD: Relative Afferent Pupillary Disorder; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; WHO: World Health Organization
Copy right Statement: Content published in the journal follows Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). © Liu H (2023)
Journal: Open Journal of Clinical and Medical Case Reports is an international, open access, peer reviewed Journal mainly focused exclusively on the medical and clinical case reports.
Citation: Liu J, Liu H. An MDT treatment of extremely proptosis caused by the recurrent meningioma. Open J Clin Med Case Rep. 2023; 1986.
Introduction
Meningiomas originate from the meningeal covering of the central nervous system and are the most common primary tumor of the central nervous system with an annual incidence of about 5 per 100,000 people. Meningiomas are usually benign and rarely have malignant manifestations [1,2].
Meningiomas are more common in women (sex ratio 2-4:1) and the incidence increases with age [3].
Its clinical manifestations vary depending on the site of involvement and the degree of tumor infiltration. Surgery and radiotherapy are currently accepted first-line treatments, but combined treatment of tumors should also be considered in refractory cases[3].
Case Presentation
A 16-year-old girl presented to us because of a huge tumor on the face for 8 years and decreased visual acuity in both eyes for 2 years. The patient had a history of nasal cranial communication tumor resections twice 10 years ago, and the postoperative pathological diagnosis was meningioma.
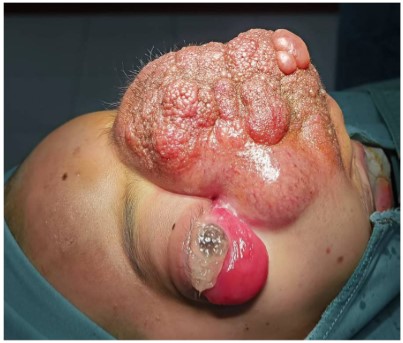
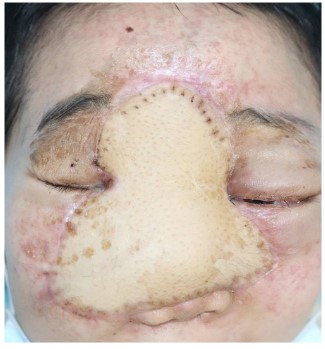
Ocular examination showed the tumor size was about 10 x 10 cm, with granular hyperplasia on the surface, accompanied by increased hair and pigmentation; The tumor is located in the middle of the face and the entire alar of the nose is completely destroyed (Figure 1); light perception was exist only in the left eye; proptosis cannot be measured due to the huge tumor size; eye movement are severely restricted in all directions in both sides; the inferior bulbar conjunctiva of both eyes is highly edematous and prolapsed outside the palpebral fissure; the corneal epithelium has a large defect area and the stromal layer is infiltrated in both eyes; the anterior chamber is normal and bilateral pupil diameter were about 5-6 mm with RAPD+ and fundus details were blurred in both sides.
CT and MRI showed that the tumor involved the anterior skull base, sphenoid and the ethmoid sinus, and invaded the inferior orbital wall, the pressure on the tumor caused the protrusion of the eyeball, and the optic nerve was pulled into a straight line.
The patient was a child with low body weight; the huge recurrent tumor has a wide range of involvement; the tumor has a rich blood supply, and the risk of anesthesia caused by intraoperative hemorrhage is high; it is difficult to determine whether the eyeball can be retracted after tumor resection; if the tumor can be completely removed, then how to reconstruct the huge defect area was faced by the surgeon.
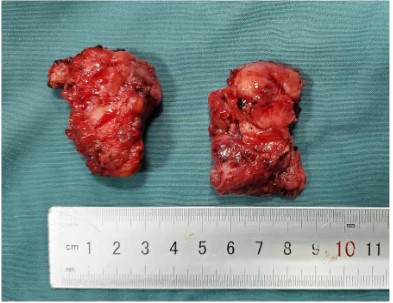
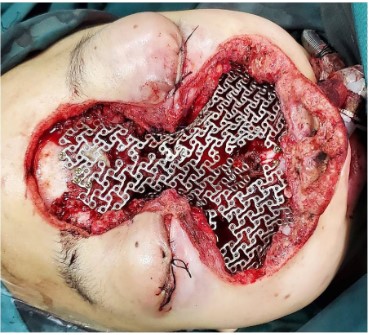
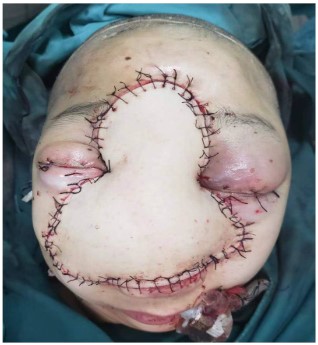
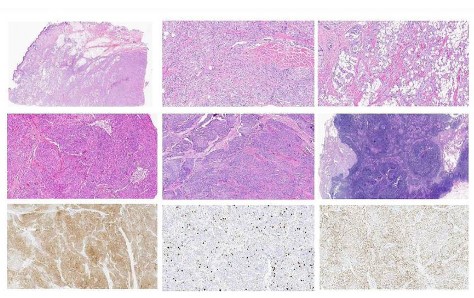
The surgical approach for this patient was designed by the MDT multidisciplinary consultation: the otolaryngologist removed the main tumor in the middle of the face and the nose, the orbital surgeon removed the orbital-infiltrating part, loosened its adhesions, and retracted the eyeball and bulbar conjunctiva into the orbit, then palpebral fissure was sutured (Figure 2). The neurosurgeon continued to remove the basicranial tumor. The defect area was reconstructed with a 3D-printed titanium mesh (Figure 3), and the skin and subcutaneous tissue were reconstructed with a free vascular pedicle flap from the lower leg to anastomose with the facial artery (Figures 4,5). The postoperative pathological diagnosis was the same as the previous - it was recurrent meningioma. HE staining showed that the tumor had invaded the skin, skin appendages, striated muscle, and lymph nodes. Immunohistochemical staining showed CD34(-), EMA (+), GFAP (-), Ki67(+,5%), PR (+), S-100(+), SSTR2(+), STAT6(-), VIM (-), CD21(-), CD35(-) (Figure 6).
The long-term follow-up showed that the skin flap survived well, and the visual acuity improved to LP in one side and HM in other side.
Discussion
Meningiomas are often diagnosed due to neurological symptoms (neurological deficits, seizures, increased intracranial pressure) or tinnitus or headache. Magnetic Resonance Imaging (MRI) is often used to make an initial diagnosis and to precisely locate and measure tumor size. The currently identified risk factors associated with meningioma development are radiation therapy and hormone intake (cyproterone acetate), and both have a dose-response relationship. Studies show that stopping hormone therapy intake even reduces tumor size [4-6].
The diagnosis of an anomalous origin of a hypoplastic right coronary artery (RCA) from the left coronary sinus with an interarterial course between the aorta and the right ventricle exit tract was made and, consequently, an assessment for surgical treatment was carried out. Evaluated by the Cardiac Surgical Team, the manipulation of the right coronary artery was rejected due to its hypoplastic nature and the clear left dominance. Surgical options were discarded and an implantable cardioverter defibrillator (ICD) was implanted as the most appropriate treatment. In the 18 months of follow-up, there were not clinical nor electrical events.
According to the 2000/2007/2016 WHO classification of meningiomas into 15 subtypes and various invasive criteria (mitotic, necrotic, cellular aspect) based on their histological appearance [7], Overall, more than 80% are grade I benign tumors [3] while atypical grade II includes 4-15% of meningiomas, and malignant grade III accounts for 1-3% [8]. While histological classification is currently the gold standard for the diagnosis and treatment of meningioma, there is still much debate about its relevance. Tumors are very heterogeneous within each grade moreover, some diagnostic criteria are vaguely defined and subject to a high interobserver bias [9-11]. The 5-year survival rate is 70% for benign meningiomas and 55% for malignant meningiomas [12], and prognostic factors include age, male gender, low Karnofsky performance status, high grade, high mitotic rate, subtotal surgical resection, and optic nerve involvement[13-16].
The classic first-line treatment for all meningiomas is surgery. However, a wait-and-see strategy is acceptable when the clinical situation permits, but requires regular observation of tumor changes [17]. Symptomatic treatments including oral or intravenous steroids help temporarily relieve symptoms by reducing peripheral edema [18]. Complete tumor resection is not always achieved intraoperatively due to tumor location and invasion of surrounding structures and brain parenchyma. Radiation therapy has become the first-line option for skull base lesions encasing vascular nerve structures such as the optic nerve sheath or cavernous sinus. If surgery is not feasible, radiation therapy may be provided alone [19]. Grade I meningiomas are usually treated with surgery or radiosurgery only, with adjuvant radiation therapy used to treat tumor remnants [20] . The 5-year recurrence rates of grade II and III meningiomas are as high as 30-40% and 50-80%, respectively [10,14]; adjuvant radiation therapy to the tumor area is also recommended along with gross resection [21]. side effects of radiotherapy and radiosurgery are usually mild but there is also evidence that radiation increases the risk of malignant transformation [22].
Long-term follow-up studies have shown that even in so-called completely resected tumors, up to 60% of tumors may recur after 15 years [10]. Various pharmacological treatment regimens have been tried, but none have been proven to be effective. Attempts at targeted therapy (anti-angiogenic molecules such as sunitinib or bevacizumab, immunotherapy or FAK inhibitors) depending on the molecular characteristics of the tumor may lead to new therapeutic directions [23,24].
Conclusion
Meningioma is the most common neurological tumor, and its treatment options and prognosis vary greatly according to different pathological features, and complete surgical resection can reduce the probability of its recurrence. A personalized treatment plan based on the patient’s clinical presentation, imaging, and pathological features should be recommended.
Acknowledgements: We gratefully acknowledge the patient and her family for their trusting and letting us report this clinical case.
References
- Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. The Lancet Neurology. 2006; 5: 1045-54.
- Zouaoui S, Darlix A, Rigau V, Mathieu-Daudé H, Bauchet F, et al. Descriptive epidemiology of 13,038 newly diagnosed and histologically confirmed meningiomas in France: 2006-2010. Neuro-Chirurgie. 2018; 64: 15-21.
- Saraf S, McCarthy BJ, Villano JL. Update on meningiomas. The oncologist. 2011; 16: 1604-1613.
- Cebula H, Pham TQ, Boyer P, Froelich S. Regression of meningiomas after discontinuation of cyproterone acetate in a transsexual patient. Acta neurochirurgica 2010; 152: 1955-1956.
- Botella C, Coll G, Lemaire JJ, Irthum B. [Intra cranial meningiomas and long term use of cyproterone acetate with a conventional dose in women. A report of two cases of tumor decrease after treatment withdrawal]. Neuro-Chirurgie. 2015; 61: 339-342.
- Bernat AL, Oyama K, Hamdi S, Mandonnet E, Vexiau D, Pocard M, et al. Growth stabilization and regression of meningiomas after discontinuation of cyproterone acetate: a case series of 12 patients. Acta neurochirurgica. 2015; 157: 1741-1746.
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica 2016; 131: 803-820.
- Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, et al. EANO guidelines for the diagnosis and treatment of meningiomas. The Lancet Oncology 2016; 17: e383-91.
- Vaubel RA, Chen SG, Raleigh DR, Link MJ, Chicoine MR, Barani I, et al. Meningiomas With Rhabdoid Features Lacking Other Histologic Features of Malignancy: A Study of 44 Cases and Review of the Literature. Journal of neuropathology and experimental neurology. 2016; 75: 44-52.
- Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. Journal of neurosurgery. 2015; 122: 4-23.
- Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. The Lancet Oncology. 2017; 18: 682-694.
- McCarthy BJ, Davis FG, Freels S, Surawicz TS, Damek DM, Grutsch J, et al. Factors associated with survival in patients with meningioma. Journal of neurosurgery. 1998; 88: 831-839.
- Pasquier D, Bijmolt S, Veninga T, Rezvoy N, Villa S, et al. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. International journal of radiation oncology, biology, physics. 2008; 71: 1388-1393.
- Durand A, Labrousse F, Jouvet A, Bauchet L, Kalamaridès M, Menei P, et al. WHO grade II and III meningiomas: a study of prognostic factors. Journal of neuro-oncology. 2009; 95: 367-375.
- Kim D, Niemierko A, Hwang WL, Stemmer-Rachamimov AO, Curry WT, Barker FG, et al. Histopathological prognostic factors of recurrence following definitive therapy for atypical and malignant meningiomas. Journal of neurosurgery. 2018; 128: 1123-1132.
- Stafford SL, Perry A, Suman VJ, Meyer FB, Scheithauer BW, Lohse CM, et al. Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clinic proceedings. 1998; 73: 936-942.
- Lee EJ, Park JH, Park ES, Kim JH. «Wait-and-See» Strategies for Newly Diagnosed Intracranial Meningiomas Based on the Risk of Future Observation Failure. World neurosurgery. 2017; 107: 604-611.
- Englot DJ, Magill ST, Han SJ, Chang EF, Berger MS, McDermott MW. Seizures in supratentorial meningioma: a systematic review and meta-analysis. Journal of neurosurgery 2016; 124: 1552-1561.
- Zhang G, Zhang Y, Zhang G, Li D, Wu Z, Wang Y, et al. Outcome and prognostic factors for atypical meningiomas after first recurrence. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia.2019; 63: 100-105.
- Noël G, Renard A, Valéry C, Mokhtari K, Mazeron JJ. [Role of radiotherapy in the treatment of cerebral meningiomas]. Cancer radiotherapie : journal de la Societe francaise de radiotherapie oncologique. 2001; 5: 217-236.
- Walcott BP, Nahed BV, Brastianos PK, Loeffler JS. Radiation Treatment for WHO Grade II and III Meningiomas. Frontiers in oncology. 2013; 3: 227.
- Yamanaka R, Hayano A, Kanayama T. Radiation-Induced Meningiomas: An Exhaustive Review of the Literature. World neurosurgery. 2017; 97: 635-44.e8.
- Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nature reviews Cancer. 2014; 14: 598-610.
- Samkari A, White J, Packer R. SHH inhibitors for the treatment of medulloblastoma. Expert review of neurotherapeutics. 2015; 15: 763-770.