Open Access, Volume 9
Idiopathic orbital inflammatory syndrome with pituitary and skull base involvement
Saad Aamir1*; Kateřina Vyhnálková2; Yamini Krishna3; Jose Gonzalez-Martin4; Gavin Cleary5; Sarah E. Coupland3; Stefan Spinty2; Shivaram Avula1
1Department of Radiology, Alder Hey Children’s Hospital, Merseyside NHS Trusts, Liverpool, UK.
2Department of Paediatric Neurology, Alder Hey Children’s Hospital, Merseyside NHS Trusts, Liverpool, UK.
3Department of Cellular Pathology, Liverpool Clinical Laboratories, Liverpool University Hospitals Foundation Trust, Liverpool, UK.
4Department of Ophthalmology, Alder Hey Children’s Hospital, Merseyside NHS Trusts, Liverpool, UK.
5Department of Rheumatology, Alder Hey Children’s Hospital, Merseyside NHS Trusts, Liverpool, UK.
Saad Aamir
Department of Radiology, Alder Hey Children’s Hospital, Merseyside NHS Trusts, Liverpool, UK.
Email: Saamir1991@gmail.com
Received : January 24, 2023,
Accepted : February 17
Published : February 20, 2023,
Archived : www.jclinmedcasereports.com
Abstract
We herein describe the case of a patient who presented with a rare diagnosis of idiopathic orbital inflammatory syndrome with a right sided orbital tumour-like mass, extra-orbital extension along the cavernous sinus to infiltrate the sella turcica and spread along the dura mater of the skull base. These sites being involved simultaneously is a rare occurrence, and as such requiring a systematic multidisciplinary diagnostic approach.
Keywords: IOIS; Skull base; Pseudo tumor.
Copy right Statement: Content published in the journal follows Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). © Aamir S (2023)
Journal: Open Journal of Clinical and Medical Case Reports is an international, open access, peer reviewed Journal mainly focused exclusively on the medical and clinical case reports.
Citation: Aamir S, Vyhnálková K, Krishna Y, Gonzalez-Martin J, Cleary G, et al. Idiopathic orbital inflammatory syndrome with pituitary and skull base involvement. Open J Clin Med Case Rep. 2023; 1984.
Introduction
Idiopathic Orbital Inflammatory Syndrome (IOIS) is an inflammatory syndrome of unknown aetiology, also known as orbital pseudotumour. It is a non-infective inflammatory condition which can affect all the anatomical structures that make up the orbit and may also extend to involve periorbital sites [1,2]. It is a rare finding in children and can have a highly variable clinical presentation ranging from a diffuse process to a specific focal target. It is diagnosed by excluding other orbital diseases with a similar presentation, such as sarcoidosis, granulomatosis with polyangiitis, Sjogren syndrome, IgG4-related disease, lympho-proliferative, or metastatic disease. Consequently, careful history taking, review of previous medical conditions, and a multidisciplinary approach is required to establish the diagnosis of IOIS [2]. We herein describe a case of a patient who presented with a unique constellation of findings indicative of IOIS, which required a multidisciplinary approach to diagnosis and manage with multi-collaborative input from speciality paediatric neurology, rheumatology, endocrinology, oncology, ophthalmology, pathology & radiology teams.
Case Presentation
We report the case of a 14-year-old female of Filipino descent, with no past medical history. She was referred to paediatric neurology for evaluation of 3-month history of right sided headaches and diplopia. On examination of extraocular movements, she had underaction of elevation of her right eye. She was also noted to have right-sided proptosis. Blood work revealed low IGF1, raised prolactin and normal sodium. The remainder of her neurological, cardiovascular, respiratory, abdominal and musculoskeletal examinations were normal.
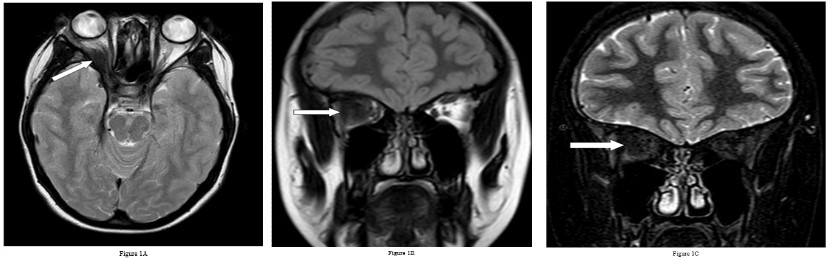
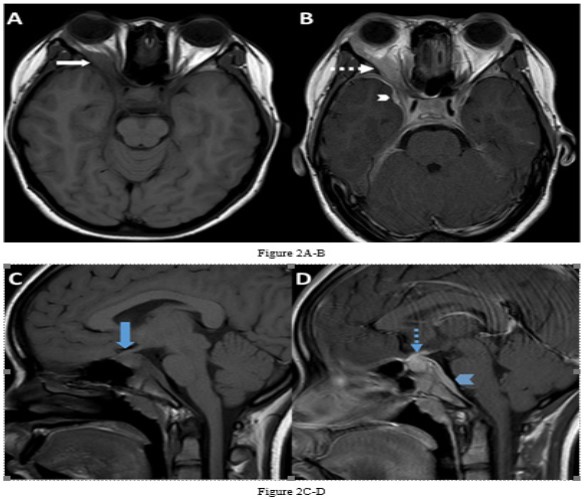
She underwent Magnetic Resonance Imaging (MRI) of her brain that identified a soft tissue lesion within the right orbit involving the posterolateral aspect and exhibited low signal on T1, T2, Fluid Attenuated Inversion Recovery (FLAIR), Short Tau Inversion Recovery (STIR) sequences, and uniform gadolinium contrast enhancement (Figures 1,2). There was no evidence of diffusion restriction. The orbital lesion was exerting mass effect on the adjacent optic nerve and displacing the adjacent extraocular muscles. The pituitary gland was enlarged on the sagittal T1-weighted sequence with loss of the normal T1 high intensity of the posterior pituitary gland. The pituitary stalk was enlarged and enhanced with gadolinium contrast, as did the pituitary gland. There was abnormal dural thickening and contrast enhancement involving the skull base from the posterior clinoid process to the clivus.
The differential diagnosis of these multiple lesions was extensive including sarcoidosis, tuberculosis, Langerhan’s cell histiocytosis, IgG4-related disease, lymphoma, neoplastic or IOIS. The initial laboratory blood tests demonstrated raised prolactin hormone level, and raised IgG, IgA, IgG1, IgG2, and IgG3 levels but normal IgG4 level. She also had normal electrolyte levels, negative connective tissue disease screen, and a negative QuantiFERON test.
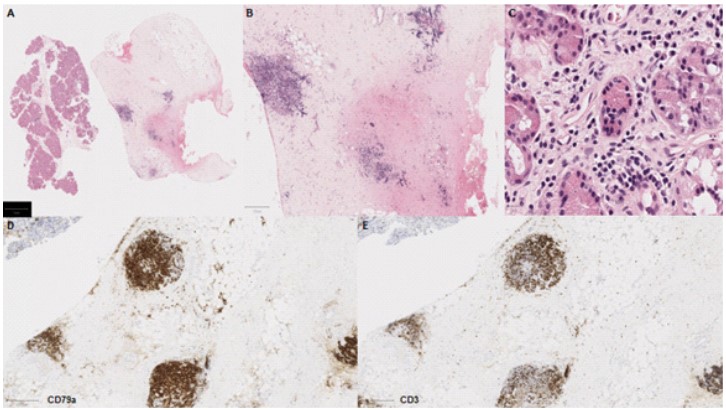
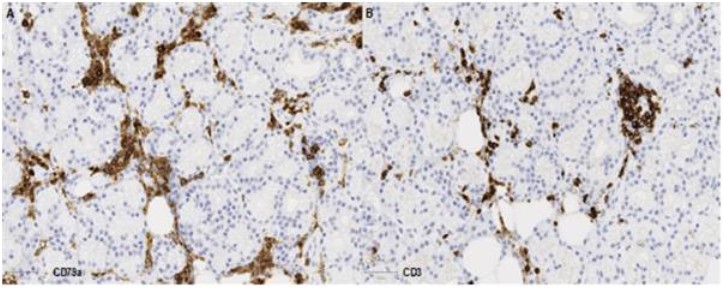
Following multidisciplinary review, a diagnostic biopsy of the right orbital lesion was taken. The orbital biopsy included lacrimal gland tissue and demonstrated presence of an oedematous collagenous stroma with patchy chronic inflammation and lymphoid follicles (Figure 3A-C), which were highlighted by equal ratio of CD3+ and CD79a+ lymphocytes (Figure 3D-E). The lacrimal gland biopsy showed collagenous stroma with a relatively dense network of CD79a+ plasma cells (Figure 4), which did not show any light chain restriction nor express IgG4. Furthermore, IGH- and IGL-gene analysis by PCR did not indicate presence of a monoclonal B-cell population, thus ruling out a lymphoma. The overall features were in keeping with IOIS. There was no evidence of granulomatous inflammation nor features to suggest Langerhan’s cell histiocytosis (CD1a and Langerin immunonegative), and no neoplastic cells.
The patient was commenced on intravenous methylprednisolone 1 g once daily (3o mg/kg capped at maximum dose 1 g) which produced a remarkable improvement in her symptoms followed by oral prednisolone 1 mg/kg at a weaning dose. To maintain steroid sparing remission the patient was also treated with subcutaneous weekly methotrexate (15 mg/m2) as long-term plan for immunomodulation.
At two weeks follow up, her visual function was normal although extraocular movement were still limited with underaction of the right lateral and superior recti muscles, and presence of a convergent strabismus in the primary position of gaze, this ocular misalignment improved in the following four weeks with resolution for the horizontal diplopia.
The patient self-stopped methotrexate after approximately 7 months due to anxiety relating to rendering COVID vaccination less effective. As her unaided vision was excellent and her squint had resolved the clinical team agreed to monitor with further follow up pending.
The diagnosis of an anomalous origin of a hypoplastic right coronary artery (RCA) from the left coronary sinus with an interarterial course between the aorta and the right ventricle exit tract was made and, consequently, an assessment for surgical treatment was carried out. Evaluated by the Cardiac Surgical Team, the manipulation of the right coronary artery was rejected due to its hypoplastic nature and the clear left dominance. Surgical options were discarded and an implantable cardioverter defibrillator (ICD) was implanted as the most appropriate treatment. In the 18 months of follow-up, there were not clinical nor electrical events.
Discussion
IOIS is a diagnosis of exclusion that requires a multi-disciplinary approach and careful correlation between the clinical, radiological and pathological findings. The clinical presentation can be acute, insidious, chronic or relapsing. Our patient presented insidiously with a prolonged headache, convergent strabismus and underaction of elevation of the right eye. Additional clinical findings to assess include periorbital erythema and edema, conjunctival congestion, ptosis, diplopia, photophobia, or ophthalmoplegia. Clinical findings are usually unilateral in adults but bilateral in children. Investigations should be informed by clinical findings blood tests with a minimum screen to include full blood count and film, electrolyte panel, C-reactive protein, erythrocyte sedimentation rate, thyroid function tests, connective tissue disease screen, angiotensin converting enzyme level, and a QuantiFERON test [2].
Radiological investigations will help to further narrow the differential diagnosis, assess extent of involvement and potential complications. Initial imaging investigations readily available include Computed Tomography (CT) and MRI studies, with orbital ultrasonography used for assessing the integrity of the eye and ocular complications such as retinal or choroidal detachments. CT and MRI studies can assess involvement of the lacrimal gland, extra ocular muscles, orbital fat, globe, presence of a mass in the orbital apex (Tolosa-Hunt syndrome subtype). CT is superior to assess underlying bone destruction or presence of calcification. MRI gives better visualisation for subtle inflammation, nerves and muscles [2,3].
MRI imaging should include the following sequences: T1-weighted, T2-weighted, Diffusion Weighted Imaging (DWI) and contrast enhanced sequences in at least 2 orthogonal planes. T1-weighted sequences will return a low to intermediate signal of the areas of inflammation, with a similar signal characteristic on the T2-weighted sequence. DWI helped to further characterise lesions which have a low T2-weighted signal, by assessing the signal from the Apparent Diffusion Coefficient (ADC) map. Malignant lesions have demonstrated a lower ADC than benign lesions, and a lymphoma lesion demonstrates a lower ADC value when compared to IOIS. Following contrast administration, enhancement in the acute stage will be uniform of the mass lesion and any associated sites of involvement, whereas in the fibrosis or sclerosing types of inflammation there will be minimal to moderate contrast enhancement [1,3,4]. The characteristic feature in IOIS is the combination of a hypointense T2 signal and enhancement suggesting an underlying fibrosis.
Besides involving the intra-orbital structures, IOIS may extend intracranially through the superior/ inferior orbital fissure or the optic canal. In our case, there was formation of an inflammatory mass lesion in the orbit with involvement of the pituitary gland and the dura along the skull base. There was extension of the intra-orbital involvement along the right sided cavernous sinus to involve the pituitary gland and associated dural layer and is rare to involve all these sites simultaneously [1,5]. An important feature on imaging in this case is the dural enhancement that connected the orbital and pituitary abnormalities suggesting a common pathology involving both regions. Dural enhancement can be subtle in some instances and need to be actively looked for.
Given the rarity of the multiple sites of involvement in our patient, the differential diagnosis following the imaging studies was between IOIS, Langerhans cell histiocytosis or an underlying neoplastic process. Although there were imaging features of IOIS, the extent of involvement was unusual and an open surgical biopsy was preferred to obtain enough tissue for histological analysis and provide a definitive diagnosis. This should be performed pre-administration of corticosteroids to not complicate histopathological diagnosis [6].
In conclusion, our case highlights a rare diagnosis of idiopathic orbital inflammatory syndrome with the presence of an inflammatory mass, infiltration of the sella turcica and dura along the skull base. This case required a systematic approach to arrive at this diagnosis with a multidisciplinary collaboration.
Learning points
• Idiopathic orbital inflammatory syndrome is a diagnosis of exclusion and requires a systematic approach to diagnose and exclude other causes of orbital inflammation.
• Computed tomography and magnetic resonance imaging studies are the gold standard investigations to assess for the intra- and extra-orbital extent of the condition.
• A multidisciplinary approach to diagnosis is recommended with histopathological confirmation of the condition if amenable to sampling.
References
- Yeşiltaş YS, Gündüz AK. Idiopathic Orbital Inflammation: Review of Literature and New Advances. Middle East Afr J Ophthalmol. 2018; 25: 71-80.
- Ronquillo Y, Patel BC. Nonspecific Orbital Inflammation. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
- Weber AL, Romo LV, Sabates NR. Pseudotumor of the orbit. Clinical, pathologic, and radiologic evaluation. Radiol Clin North Am. 1999; 37: 151-168.
- Patnana M, Sevrukov AB, Elsayes KM, Viswanathan C, Lubner M, et al. Inflammatory pseudotumor: the great mimicker. AJR Am J Roentgenol. 2012; 198: W217-27.
- Olmos PR, Falko JM, Rea GL, Boesel CP, Chakeres DW, et al. Fibrosing pseudotumor of the sella and parasellar area producing hypopituitarism and multiple cranial nerve palsies. Neurosurgery. 1993; 32: 1015-1021.
- Mombaerts I, Rose GE, Garrity JA. Orbital inflammation: Biopsy first. Surv Ophthalmol. 2016; 61: 664-669.