Open Access, Volume 9
Fulminant clostridioides difficile colitis in an obese patient with previous bilio-pancreatic diversion
Virginia Laurenti1; Davide Zulian1,2; Vincenzo Belsito1,3; Simone Giudici1,2; Bruno Branciforte2; Anastasia Baire1,2; Andrea Brocchi2; Paola Spaggiari1,3; Martina Ceolin2; Daniele Del Fabbro2*
1Department of Biomedical Sciences, Humanitas University, Via Rita Levi Montalcini, 4, 20090 Pieve Emanuele (MI), Italy. 2Division of Trauma and Emergency Surgery, Department of Surgery, IRCCS Humanitas Research Hospital, via Manzoni 56, 20089 Rozzano, Milan, Italy. 3Department of Pathology, IRCCS Humanitas Research Hospital, via Manzoni 56, 20089 Rozzano, Milan, Italy.
Daniele Del Fabbro
Division of Trauma and Emergency Surgery, Department of Surgery, IRCCS Humanitas Research Hospital,
Via Manzoni, 56, 20089, Rozzano, Milan, Italy.
Email: daniele.del_fabbro@cancercenter.humanitas.it
Received : November 28, 2022,
Accepted : January 20, 2023
Published : January 31, 2023,
Archived : www.jclinmedcasereports.com
Abstract
Clostridioides difficile infection is a common nosocomial infection, generally related to prolonged antibiotic therapy. Over the past decade, increasing rates of healthcare associated C.difficile colitis have been observed. Most cases are treated medically with antibiotic and supportive therapy. The choice of treatment must be guided by the severity of disease. Although most patients develop a mild or moderate infection, a small percentage manifest a severe or fulminant colitis.
We hereby describe a case of recurrent C.difficile infection in a 48-year-old patient who had previously undergone bilio-pancreatic diversion, presenting as severe colitis determining septic shock. After medical therapy failure he has been treated with total colectomy and an open abdomen approach for the first 48h post-operatively.
In severely ill patients who fail to respond to medical therapy, surgical treatment must be promptly considered and may improve survival.
Keywords: C.difficile; Clostridioides difficile; Pseudomembranous colitis; Total colectomy; Bilio-pancreatic diversion.
Abbreviations: CD: Clostridioides difficile; CDI: Clostridioides difficile infection; CDC: Clostridioides difficile colitis; CT: Computed tomography; IV: Intravenous; CRP: C- Reactive Protein; PCT: Procalcitonine; RYGB: Roux-en-Y gastric bypass; VSG: vertical sleeve gastrectomy; PPI: Proton Pump Inhibitors.
Copy right Statement: Content published in the journal follows Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). © Fabbro DD (2023)
Journal: Open Journal of Clinical and Medical Case Reports is an international, open access, peer reviewed Journal mainly focused exclusively on the medical and clinical case reports.
Citation: Laurenti V, Zulian D, Belsito V, Giudici S, Fabbro DD, et al. Fulminant clostridioides difficile colitis in an obese patient with previous bilio-pancreatic diversion. Open J Clin Med Case Rep. 2023; 1970.
Introduction
Clostridioides (formerly Clostridium) difficile infection (CDI) is a common nosocomial infection, with an estimated incidence of 73 per 100,000 patients residing in hospitals or health-care facilities [1].
It is generally related to prolonged antibiotic therapy, with subsequent disruption of the intestinal microbiome and selection of resistant and more virulent strains of the germ.
Colonization of the gut occurs via the fecal-oral route by ingestion of spores. After exposure to C. difficile, some patients remain asymptomatic. Asymptomatic colonization has been defined as a positive stool culture for C. difficile in the absence of diarrhea. The carriage rate of C. difficile is about 3% among healthy adults, while the percentage of asymptomatic carriers among patients residing in hospitals or health care facilities reaches 10% [2].
CDI has been defined as follows: the presence of diarrhea and a positive C. difficile cytotoxin assay or toxigenic culture, the presence of diarrhea without an alternative explanation and an endoscopic diagnosis of pseudomembranes, or a pathological diagnosis of C. difficile infection.
Although both patients with clinical infection and asymptomatic ones represent the bacteria reservoir, transmission is more likely to occur in presence of CD-associated diarrhea [3].
More often, C. difficile infection results in the development of a mild or moderate infection. However, a small but significant percentage manifest a severe or fulminant disease. C. difficile colitis (CDC) is classified as fulminant when associated with hypotension or shock, ileus, or megacolon [4]. Variability in host risk factors may explain the wide spectrum of symptoms and clinical course.
A considerable proportion of patients (ranging from 10% to 50%) with a first diagnosis of CDI develop at least one recurrence [5], defined as occurring within 8 weeks from the previous episode [6]. Recurrences are more likely due to treatment failure than reinfection with a different strain of the microorganism [7]. In that subset of severely ill patients who progress or fail to respond to medical therapy, surgical treatment must be promptly considered.
In addition to antibiotic use, obesity and bariatric surgeries have been described in several studies as independent risk factors for CDI and CDI severity, possibly due to underlying intestinal dysbiosis [8,9].
Hereby, we describe a case of recurrent CDI in a patient who had previously undergone bilio-pancreatic diversion, presenting as severe colitis determining septic shock and successfully treated with total colectomy after medical therapy failure.
Case Presentation
A 48-years old man presented to the emergency department complaining of abdominal pain, diarrhea, and nausea. He reported having from 3 to 4 loose stools per day for the past three days, associated with elevated fever and chills. One month before, the patient had undergone cholecystectomy; post-operative course was complicated by a perihepatic abscess, addressed with a 3-weeks course of amoxicillin treatment. Furthermore, he reported having had a CDI six weeks earlier, treated with oral vancomycin and followed by symptoms resolution.
The patient’s medical history included dyslipidemia and 3 years earlier he underwent bariatric bilio-pancreatic diversion.
On admission, the patient was tachycardic at 110 beats/min, slightly hypotensive with blood pressure of 105/70 mmHg and feverish, body temperature measuring 39°C. Abdominal examination revealed diffuse tenderness and distension without peritoneal signs.
Initial laboratory test results included white count 28100/mm3 with 90% of neutrophiles, CRP 8,82 mg/dL, PCT 0,84 ng/mL. The first and only set of blood cultures drawn remained negative. Stool tested positive for Clostridium difficile toxin B by nucleic acid amplification test.
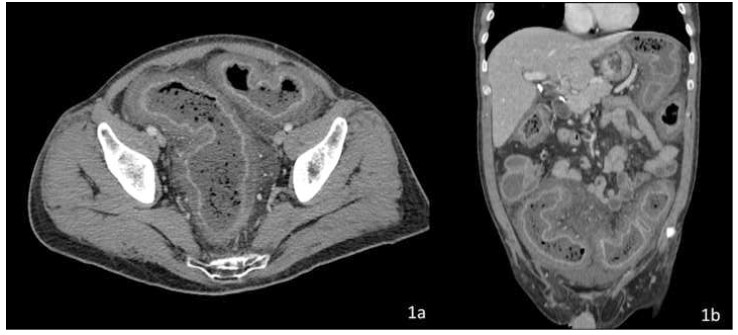
Computed tomography (CT) with IV and oral contrast revealed severe, diffuse colonic wall thickening, pericolic fat stranding and marked enhancement of the colic mucosa and submucosa secondary to hyperemia (Figure 1).
In the emergency department, the patient was started on fluid resuscitation and broad-spectrum antimicrobial therapy with piperacillin-tazobactam 4.5 g IV and oral vancomycin, the first dose of which was given less than 1 hours after the admission. Given the poor clinical and biochemical response to the initial treatment, it was subsequently escalated to IV Meropenem 1g 3 times daily, Anidulafungin 100 mg 3 times daily IV Vancomycin 500 mg and Metronidazole 500 mg.
On re-evaluation, the patient showed a worsening abdominal exam and progressive hemodynamic instability with need for vasopressor support despite aggressive fluid resuscitation. Additionally, he developed atrial fibrillation, oliguria, and metabolic acidosis. The new blood tests showed a further increase in leucocytes and CRP.
Given the deteriorating clinical conditions, an emergency exploratory laparotomy was indicated, and the patient was taken to the operatory room 48 h after the admission.
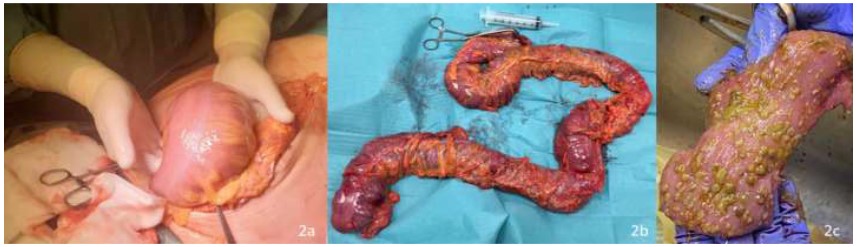
Intraoperatively, a modest amount of citrine fluid was found and sent for microbiological examination. The whole colon and the proximal rectum appeared severely dilated and edematous, with a greyish serosa. No signs of perforation or necrosis were evident. A laparotomic total colectomy was performed by dividing the terminal ileum, resecting the entire colon, and dividing it at the rectosigmoid junction, leaving a blind, closed-off pouch of rectum in the abdomen (Figure 2). An open abdomen approach was used due to the concurrent small bowel distension and the patient’s hemodynamic instability, to decrease risk for postoperative intra-abdominal hypertension. 48 hours later, after the patient’s stabilization and the subsiding of the edema, a terminal ileostomy and abdominal wall closure was performed. The postoperative course was uneventful, and the patient was discharged on postoperative day 10.
In consideration of the patient’s previous bilio-pancreatic diversion and its possible risks of malnutrition and diarrhea, especially in this new setting, he was evaluated during hospitalization by the bariatric surgeons and their dietetics service. He will continue the follow-up with the bariatrics team and an eventual reversal surgery might be needed.
Pathologic microscopic evaluation of the specimen confirmed the diagnosis of pseudomembranous colitis (Figure 3).
Discussion
Over the past decades, increasing rates of healthcare associated CDC have been observed, particularly in the elderly.
Both pathogen and host factors play a role in the development and the severity of CDI.
The best-described C. difficile virulence factors are toxins A and B, capable of leading to colonocyte death and disruption of the intestinal barrier.
Among the known host-related risk factors for CDI there are age >65 years, immunosuppression, malnutrition, increased severity of underlying illness, prior hospitalization, use of feeding tubes, use of proton-pump inhibitors, prolonged hospital stays and antibiotic use within the past 6 to 8 weeks [10].
Even though any antibiotic can potentially predispose to CDI, clindamycin, fluoroquinolones, and broad-spectrum penicillin are the most frequently involved [11].
As additional risk factors both obesity and bariatric surgery, particularly Roux-en-Y gastric bypass (RYGB) rather than vertical sleeve gastrectomy (VSG), have been described as independently associated with CDI development [8]. This association can be explained by the intestinal microbiome alterations accompanying obesity and bariatric interventions [9]. In particular, the bilio-pancreatic diversion, implying a shortened oral-colonic transit and a frequent PPI use, could lead, potentially even more than RYGB, to intestinal dysbiosis and increased rates of CDI.
Most cases of CDI are treated medically with antibiotic and supportive therapy. Anyway, the choice of treatment must be guided by the severity of disease.
According to current guidelines, first line antibiotics regimens in non-fulminant cases should include either oral fidaxomicin or oral vancomycin, the former to be preferred in case of severe or recurrent disease. For fulminant CDC, recommended antibiotic therapy include oral (or per nasogastric tube) vancomycin plus parenteral metronidazole [12].
Critically ill patients and those not responding to adequate medical treatment should be considered for surgical treatment. Colonic perforation, full-thickness ischemia, abdominal hypertension or abdominal compartment syndrome, organ failure and worsening of clinical conditions despite medical therapy constitute absolute indications for surgery [13].
Currently accepted options of surgical treatment include total abdominal colectomy and diverting loop ileostomy with colonic lavage. Excepted for those cases presenting with abdominal compartment syndrome, colonic perforation, or colonic necrosis, and therefore requiring a total colectomy, none of the two procedures is considered superior to the other. Although diverting loop ileostomy with colonic lavage is a less invasive procedure with higher rates of stoma reversal [14,15], a total colectomy allows the resection of the inflamed colon and therefore a more likely resolution of systemic inflammation and critical illness.
For this reason, total abdominal colectomy is the preferred operation for patients with fulminant CDC who fail medical management.
In conclusion, C.difficile infection is a common and increasing nosocomial entity. The severity of disease must guide the choice of treatment. Most patients develop a mild or moderate infection, that is usually treated medically with antibiotic and supportive therapy. A small percentage manifest a severe or fulminant colitis.
It is imperative to include this condition as a potential etiology in febrile long-stay patients, treated with long term antibiotic regimens, who present leukocytosis, diarrhea, and severe colonic distention. A prompt surgical consultation may improve patient survival in such cases.
References
- Guh AY, Mu Y, Winston LG, Johnston H, Olson D, et al. Emerging Infections Program Clostridioides difficile Infection Working Group. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N Engl J Med. 2020; 382: 1320-1330.
- Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015; 110: 381-391.
- Kong LY, Eyre DW, Corbeil J, Raymond F, Walker AS, et al. Clostridium difficile: Investigating Transmission Patterns Between Infected and Colonized Patients Using Whole Genome Sequencing. Clin Infect Dis. 2019; 68: 204-209.
- McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018; 66: e1-e48.
- Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008; 70: 298-304.
- McDonald LC, Coignard B, Dubberke E, Song X, Horan T, et al. Ad Hoc Clostridium difficile Surveillance Working Group. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007; 28: 140-145.
- Figueroa I, Johnson S, Sambol SP, Goldstein EJC, Citron DM, et al. Relapse versus reinfection: recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012; 55: S104-109.
- Hussan H, Ugbarugba E, Bailey MT, Porter K, Needleman B, et al. The Impact of Bariatric Surgery on Short Term Risk of Clostridium Difficile Admissions. Obes Surg. 2018; 28: 2006-2013.
- Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, et al. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015; 22: 228-238.
- Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011; 365: 1693-703.
- Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. 2013; 57: 2326-32.
- Johnson S, Lavergne V, Skinner AM, Gonzales-Luna AJ, Garey KW, et al. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin Infect Dis. 2021; 73: 755-757.
- Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P. West Midlands Research Collaborative. Systematic review and metaanalysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg. 2012; 99: 1501-1513.
- Felsenreich DM, Gachabayov M, Rojas A, Latifi R, Bergamaschi R. Meta-analysis of Postoperative Mortality and Morbidity After Total Abdominal Colectomy Versus Loop Ileostomy With Colonic Lavage for Fulminant Clostridium Difficile Colitis. Dis. Colon Rectum. 2020; 63: 1317-1326.
- Neal MD, Alverdy JC, Hall DE, Simmons RL, Zuckerbraun BS. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann. Surg. 2011; 254: 423-429.