Open Access, Volume 9
Dramatic response of a negative PD-L1 expression and TMB low pulmonary sarcomatoid carcinoma to sintilimab combined with anlotinib
Xiaoli Lione1; Na Maone1; Shulan Haoone1; Yizhe Zhangone1; Yuan Tantwo2; Qianqian Duantwo2; Qin Zhangtwo2; Fengchao Chaione1; Qimeng Sunone1; Likun Liuone1*
1Department of Oncology, Shanxi Traditional Chinese Medical Hospital,China; Shanxi University of Traditional Chinese Medicine,
China.
2The Medical Department, Jiangsu Simcere Diagnostics Co., Ltd; Nanjing Simcere Medical Laboratory Science Co., Ltd; The State
Key Lab of Translational Medicine and Innovative Drug Development, Jiangsu Simcere Diagnostics Co., Ltd; Building 5, No. 699-18 Xuanwu Avenue, Xuanwu District, Nanjing, Jiangsu Province, China.
Likun Liuone
Shanxi Traditional Chinese Medical Hospital, 46 West Bingzhou Street, Taiyuan City, Shanxi Province,
China.
Email: llkun133@126.com
Received : December 10, 2022,
Accepted : January 17, 2023
Published : January 20, 2023,
Archived : www.jclinmedcasereports.com
Abstract
Pulmonary Sarcomatoid Carcinoma (PSC) is a rare and highly malignant subtype of Non-Small-Cell Lung Cancer (NSCLC). In contrast to other types of lung cancers, recent retrospective studies demonstrated that PSC always shows frequent genetic mutations, high TMB and high PD-L1 expression which indicates these patients might derive survival benefits from immune checkpoint inhibitors. In this case report, we firstly present a 76-year-old PSC patient with PD-L1 negative (TPS<1%), TMB low (4.3 Mut/Mb) and MSS who achieving extremely long PFS (25 months) after Sintilimab combined Anlotinib treatment. Moreover, the patient had no immune-related side effects during the treatment. In summary, this PSC patient with low TMB and negative PD-L1 expression who benefits from immuno-combination therapy provides a new therapeutic perspective for PSC. And further clinical and fundamental studies are need.
Keywords: Pulmonary sarcomatoid carcinoma; Sintilimab; Anlotinib; PD-L1 negative; TMB-Low.
Copy right Statement: Content published in the journal follows Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). © Liuone L (2023)
Journal: Open Journal of Clinical and Medical Case Reports is an international, open access, peer reviewed Journal mainly focused exclusively on the medical and clinical case reports.
Citation: Lione X, Maone N, Haoone S, Zhangone Y, Tantwo Y, Duantwo Q, Liuone L, et al. Dramatic response of a negative PD-L1 expression and TMB low pulmonary sarcomatoid carcinoma to sintilimab combined with anlotinib. Open J Clin Med Case Rep. 2023; 1969.
Introduction
Pulmonary Sarcomatoid Carcinoma (PSC) is a rare NSCLC subtype characterized by poorly differentiated tumors containing sarcoma or sarcoma-like elements, rapid tumor growth, high level of blood-vessel invasion, and epithelial-to-mesenchymal transition, which worse prognosis is well known [1]. Except MET exon 14 alterations and other oncogene mutations, PSC commonly harbored high tumor mutational burden (TMB) and high level of PD-L1 expression [2]. It was widely accepted that Programmed Death-Ligand 1 (PD-L1) positive, Tumor Mutational Burden-High (TMB-H) or Microsatellite Instability-High (MSI-H) tumors are prone to better treatment response to immune checkpoint blockade. A retrospective study of PSC monotherapy immunotherapy showed the objective response rate (ORR) was 58.8% in PSC patients with PD-L1 positive expression and 0% in the single PD-L1 negative patient, with the same trend observed for disease control rate (76.5% in PD-L1 positive versus 0% in PD-L1 expression). Therefore, PD-L1 positive PSC patients are more likely to benefit from Immune Checkpoint Inhibitors (ICIs).
Sintilimab is a fully human IgG4 monoclonal antibody that binds to programmed cell death receptor-1 (PD-1), thereby blocking the interaction of PD-1 with its ligands (PD-L1 and PL-L2) and consequently helping to restore the endogenous antitumor T-cell response [2,3]. It has been shown excellent clinical benefits in the treatment of relapsed or refractory Hodgkin’s lymphoma [4] and NSCLC [5]. Anlotinib is a multitarget tyrosine kinase inhibitor. It was originally designed to inhibit VEGFR2/3, FGFR1–4 with high affinity [6]. Clinical trials have indicated that Anlotinib could prolong the progression-free survival (PFS) of patients with NSCLC [7], medullary thyroid carcinoma (MTC) and metastatic renal cell carcinoma (mRCC) [8].
Here, we firstly report an unusual PD-L1 negative and TMB low, and microsatellite stable (MSS) pulmonary sarcoma patient who achieved a good response to immune checkpoint blockade and achieved extremely long PFS (25 months). Our study reminds a new therapeutic perspective for PSC who has low TMB and negative PD-L1. In addition, further clinical and fundamental studies are needed.
Case Presentation
A 76-year-old man went to our hospital with hoarse voice on July 15, 2019. He suffered from hypertension for more than 10 years, with no history of smoking, heart disease, diabetes, cerebrovascular disease. The chest Computed Tomography (CT) scan revealed irregular soft tissue masses at (Figure 2A). The size of the mass is about 9 x 6.6 cm, and the CT value is 37 HU. The pathological results indicated that CK (-) Desmin (-), Vimentin (+), Ki-67 (10%), TTF-1 (-), CD34 (+). Morphologically the tumor had the typical appearance of a spindle cell neoplasm, which was diagnosed as sarcomatoid carcinoma. After 2 months of oral Chinese medicine treatment, the patient developed facial swelling, cough, wheezing, and chest constriction on November 21, 2019.
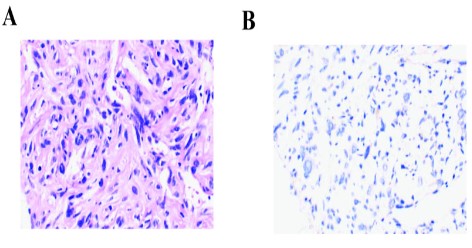
Re-examination of CT scan showed that the apical bronchus of the left upper lobe was not seen, but irregular soft tissue masses were seen, the size was about 12.5 x 10.7 cm, and the CT value was about 40 HU (Figure 2B). To seek precise treatment, next-generation sequencing (NGS) of 556 cancer-related genes panel analysis was performed. The results showed that CNV of JAK2 and PDCD1LG2 were 4 and 6 respectively, MYOD mutation and other somatic mutations were shown in supplementary Table 1. Moreover, the TMB, defined as the total number of non-synonymous somatic, coding, base substitution, and indel mutations per megabase (Mb) of genome, was 4.3 mutations/Mb. After that, PD-1 expression analysis showed that TPS <1% (clone number 22C3 (DAK0) antibody) (Figure 1). Besides that, microsatellite analysis was demonstrated that Microsatellite Stable (MSS), other potential predictive biomarkers related to immunotherapy were not found.
The patient refused chemotherapy and was given Sintilimab injection 200 mg ivgtt d1 combined with Anlotinib 8 mg/time, once a day, oral d1-14 q21d treatment on December 9, 2019. On January 2, 2020, the patient developed tight chest suffocation and back to examination. The pleural effusion on the left was more than before, and the pleural effusion on the right was newer than before. Pleural effusion will be punctured and drained, and about 5000 mL of pleural fluid will be drained, which is bloody pleural effusion. On January 10, January 17, and February 19, 2020, the patient was given cisplatin 50 mg thoracic cavity chemotherapy three times respectively, and the pleural effusion gradually decreased. Then, the patient was continuously treated with Sintilimab combined with Anlotinib. CT re-examination on February 14, 2020 showed that the mass was about 10.7 to 6.6 cm, the left pleural effusion was slightly reduced (during the drainage period), and the effect was evaluated as PR (Figure 2B). The patient suffered from fatigue due to nausea and vomiting on March 15, 2020 and Anlotinib was change to 8 mg/time oral d1-14 q21d treatment from March 23, 2020 every other day, after that the nausea was remission. A CT examination performed on April 25, 2020 revealed a soft tissue mass in the left hilar area, about 10.6 x 4.8 cm in size, and the left pleural effusion was slightly smaller than before (Figure 2B). The evaluation was to maintain PR. The re-examination of chest CT on July 28, 2020 showed that the size of the left hilar soft tissue mass was about 8.8 cm to 3.8 cm, and the left pleural effusion was less than before, and the effect was to maintain PR (Figure 2B). At the last time of CT examination, May 27, 2021, clinical evaluation was maintained PR (Figure 2B). The patient had no extremely immune-related side effects during the treatment and the PS score was 0. By the time of submission, the patient had no discomfortable symptom, and we will continue to follow up.
Table 1: somatic mutations in this patient.
| Gene | Mutation | Mutation abundance/copy number |
|---|---|---|
| MYOD1 | c.G859A | 0.59% |
| NTRK1 | c.G1520A | 0.59% |
| RNF43 | c.C1759T | 0.58% |
| CEBPA | c.C470G | 0.73% |
| CEBPA | c.C2103A | 0.58% |
| TEK | c.G2744A | 0.88% |
| JAK2 | CNV | 6 |
| PDCD1LG2 | CNV | 4 |
Discussion
Although pulmonary sarcomatoid carcinoma only accounts for 0.3–3.0% of all lung malignancies, the efficacy of current treatment options for advanced PSC is limited because of resistance to chemotherapy and low responsiveness to radiotherapy which result in these patients have poorer prognoses [9]. Recently, a genomic study of 125 PSCs has identified that PSC had recurrent targetable genes listed in the NCCN NSCLC guidelines (MET [13.6%], EGFR [8.8%], BRAF [7.2%], HER2 [1.6%], and [RET 0.8%]) and high TMB (43% PSC, 10Mut/Mb). Besides that, investigations have shown that PSC often expresses higher level of PD-L1 expression than other subtypes of NSCLC ranging from 25% to 90% [10,11]. In present, high expression of PD-L1, mismatch repair deficiency (dMMR)/MSI-H, and high TMB have been proved good predictive biomarker for immunotherapy in many cancers type. The result of KEYNOTE - 042 suggested patients with higher PD-L1 expression are associated with superior OS and higher ORR (objective response rate [12]. Some previous retrospective studies also demonstrated that patients with PD-L1 positive PSC exhibited higher response rates and prolonged overall survival under ICIs treatment than PD-L1 negative subgroup (1). When it comes to TMB, it is well known that KEYNOTE - 158 study have prospectively proved that TMB-H group had higher ORR than the counterpart (29% vs. 6%) which drove FDA approved the pembrolizumab for TMB-H (TMB≥10 Mut/Mb) solid tumors [13]. Based on these evidences, PSC patients are likely to be very good candidates for ICIs therapy. While in the clinical scenario, it was seen that some sporadic PD-L1 negative expression cases were also sensitive to ICIs treatment [14,15]. That is, the predictive power of these factors remains unclear, because of the spatiotemporal heterogeneity of each tumor [16]. The complexity of the choice of PD-L1 detection method and cut-off value of TMB further contributes to the inaccuracy of these predictors, and the predictive biomarkers may need more exploration.
In this case, although the PSC patient had negative PD-L1 expression, low TMB and MSS, he achieved persistent tremendous long PFS (25 months) to ICI combine Anlotinib. It indicated that similar PSC patients would benefit from immunotherapy. Many clinical trials have shown improved anti-cancer efficacy and prolonged survival following the addition of anti-angiogenic agents to ICIs. Studies have revealed that anti-angiogenesis and ICIs therapy can reprogram the tumor milieu from an immunosuppressive to an immune permissive microenvironment, and mutually enhance the antitumor effect [17]. In a clinical trial of Sintilimab combined with Anlotinib for advanced NSCLC, in the PD-L1 negative subgroup, sixteen patients achieved PR and 2 patients (25%) achieved SD with an ORR of 75% and a DCR of 100% [18]. Even though lack of PSC patient subgroup, that study also gave us inspiring experience in this PSC treatment. However, there are also some limitations in our present study. Firstly, this is only a one-patient case report and more cases are needed to analyze the correlation survival benefit of PSC patients with ICIs treatment. Secondly, there was no RNA-related analysis to decipher the association between the tumor immune microenvironment and immune response in the patient due to the inadequate specimen. In summary, although high TMB, MSI-H and high PD-L1 expression is associated with good efficacy of immunotherapy, the PSC patient with low TMB, MSS and negative PD-L1 expression have a great response to ICI combining Anlotinib. It provides a new idea for the treatment of pulmonary sarcoma, but it needs to be further confirmed by large prospective studies.
Declarations
Data availability statement: All data generated or analyzed during this study are included in this article.
Ethics statement: The authors’ institution does not require ethical approval for the publication of a single case report. Written informed consent was obtained from the patient.
Availability of data and materials: All data generated or analysed during this study are included in this published article [and its supplementary information files].
Authors’ contributions: XLL, Yuan T and QQD prepared the manuscript and the literature search; LKL reviewed and edited the manuscript; SLH, NM and FCC treated and observed the patient; YZ Zhang performed the histopathological, immunohistochemical examinations. QZ performed bioinformatics analysis and mapping. All authors read and approved the final manuscript.
Conflict of interest: Yuan Tan, Qianqian Duan and Qin Zhang are employed by the company Jiangsu Simcere Diagnostics Co., Ltd The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments: We thank Dr. Chuang Qi, Dr. Wanglong Deng and Dr. Guanghua Lu, and Mr. Ran Ding from Jiangsu Simcere Diagnostics for their kind assistance.
References
- Domblides C, Leroy K, Monnet I, Mazières J, Barlesi F, et al. Efficacy of Immune Checkpoint Inhibitors in Lung Sarcomatoid Carcinoma. J Thorac Oncol. 2020; 15: 860-866.
- Jiao Y, Liu M, Luo N, Guo H, Li J. Successful treatment of advanced pulmonary sarcomatoid carcinoma with the PD-1 inhibitor toripalimab: A case report. Oral Oncol. 2021; 112: 104992.
- Hoy SM. Sintilimab: First Global Approval. Drugs. 2019 ; 79 : 341-346.
- Shi Y, Su H, Song Y, Jiang W, Sun X, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2019; 6: e12-e19.
- Yang Y, Wang Z, Fang J, Yu Q, Han B, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: a Randomized, Double-Blind, Phase 3 Study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol. 2020; 15: 1636-1646.
- Chi Y, Fang Z, Hong X, Yao Y, Sun P, et al. Safety and Efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in Patients with Refractory Metastatic Soft-Tissue Sarcoma. Clin Cancer Res. 2018; 24: 5233-5238.
- Han B, Li K, Wang Q, Zhang L, Shi J, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2018; 4: 1569-1575.
- Zhou AP, Bai Y, Song Y, Luo H, Ren XB, et al. Anlotinib Versus Sunitinib as First-Line Treatment for Metastatic Renal Cell Carcinoma: A Randomized Phase II Clinical Trial. Oncologist. 2019; 24: e702-e708.
- Maneenil K, Xue Z, Liu M, Boland J, Wu F, et al. Sarcomatoid Carcinoma of the Lung: The Mayo Clinic Experience in 127 Patients. Clin Lung Cancer. 2018; 19: e323-e333.
- Babacan NA, Pina IB, Signorelli D, Prelaj A, Garassino MC, Tanvetyanon T. Relationship Between Programmed Death Receptor-Ligand 1 Expression and Response to Checkpoint Inhibitor Immunotherapy in Pulmonary Sarcomatoid Carcinoma: A Pooled Analysis. Clin Lung Cancer. 2020; 21: e456-e463.
- Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol. 2013; 8: 803-805.
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, et al. KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019; 393: 1819-1830.
- Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020; 21:1353-1365.
- Zhang F, Zhang J, Zhao L, Zhai M, Zhang T, et al. A PD-L1 Negative Advanced Gastric Cancer Patient with a Long Response to PD-1 Blockade After Failure of Systematic Treatment: A Case Report. Front Immunol. 2021; 12: 759250.
- Zhong R, Zhang Y, Chen D, Cao S, Han B, Zhong H. Single-cell RNA sequencing reveals cellular and molecular immune profile in a Pembrolizumab-responsive PD-L1-negative lung cancer patient. Cancer Immunol Immunother. 2021; 70: 2261-2274.
- Zhou KI, Peterson B, Serritella A, Thomas J, Reizine N, et al. Spatial and Temporal Heterogeneity of PD-L1 Expression and Tumor Mutational Burden in Gastroesophageal Adenocarcinoma at Baseline Diagnosis and after Chemotherapy. Clin Cancer Res. 2020; 26: 6453-6463.
- Song Y, Fu Y, Xie Q, Zhu B, Wang J, Zhang B. Anti-angiogenic Agents in Combination With Immune Checkpoint Inhibitors: A Promising Strategy for Cancer Treatment. Front Immunol. 2020; 11: 1956.
- Chu T, Zhong R, Zhong H, Zhang B, Zhang W, et al. Phase 1b Study of Sintilimab Plus Anlotinib as First-line Therapy in Patients With Advanced NSCLC. J Thorac Oncol. 2021; 16: 643-652.